Acoustic separation and concentration of exosomes for nucleotide detection: ASCENDx
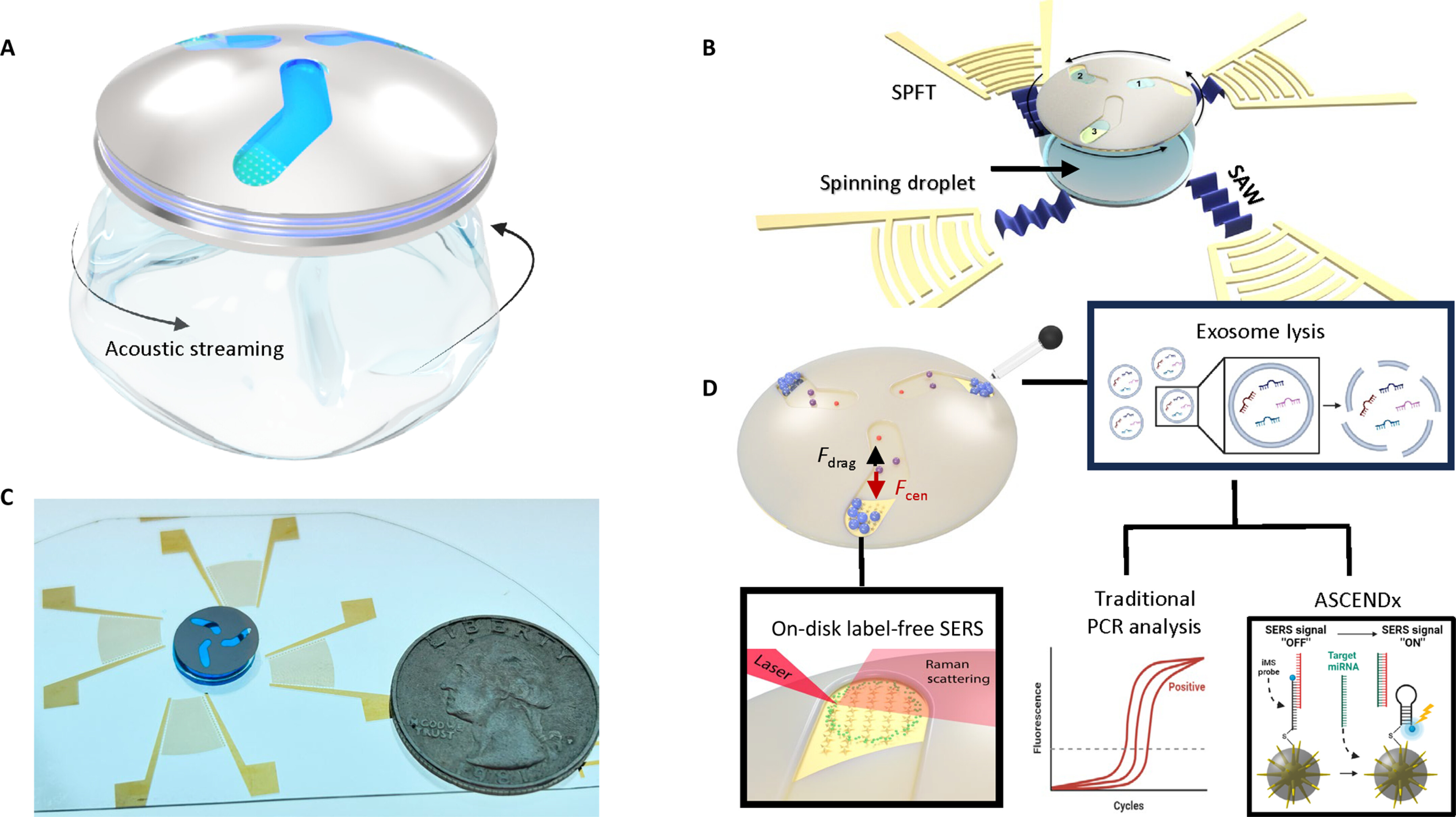
Figure: Operating mechanism of the ASCENDx platform.
Efficient isolation and analysis of exosomal biomarkers hold transformative potential in biomedical applications. However, current methods are prone to contamination and require costly consumables, expensive equipment, and skilled personnel. Here, we introduce an innovative spaceship-like disc that allows Acoustic Separation and Concentration of Exosomes and Nucleotide Detection: ASCENDx. We created ASCENDx to use acoustically driven disc rotation on a spinning droplet to generate swift separation and concentration of exosomes from patient plasma samples. Integrated plasmonic nanostars on the ASCENDx disc enable label-free detection of enriched exosomes via surface-enhanced Raman scattering. Direct detection of circulating exosomal microRNA biomarkers from patient plasma samples by the ASCENDx platform facilitated a diagnostic assay for colorectal cancer with 95.8% sensitivity and 100% specificity. ASCENDx overcomes existing limitations in exosome-based molecular diagnostics and holds a powerful position for future biomedical research, precision medicine, and point-of-care medical diagnostics.
Cellular Immunity Analysis by Modular Acoustofluidic Platform: CIAMAP

Figure: Schematic of CIAMAP-facilitated cell pairing and immunity mechanism exploration.
The study of molecular mechanisms at the single-cell level holds immense potential for enhancing immunotherapy and understanding neuroinflammation and neurodegenerative diseases by identifying previously concealed pathways within a diverse range of paired cells. However, existing single-cell pairing platforms have limitations in low pairing efficiency, complex manual operation procedures, and single-use functionality. Here, we report a multiparametric cellular immunity analysis by a modular acoustofluidic platform: CIAMAP. This platform enables users to efficiently sort and collect effector-target (i.e., NK92-K562) cell pairs and monitor the real-time dynamics of immunological response formation. Furthermore, we conducted transcriptional and protein expression analyses to evaluate the pathways that mediate effector cytotoxicity toward target cells, as well as the synergistic effect of doxorubicin on the cellular immune response. Our CIAMAP can provide promising building blocks for high-throughput quantitative single-cell level coculture to understand intercellular communication while also empowering immunotherapy by precision analysis of immunological synapses.
Acoustofluidic multimodal diagnostic system for Alzheimer's disease

Figure: Schematic illustration of the acoustofluidics-based diagnostic system (ADx) for Alzheimer's disease. Liquid biopsy is employed to test AD patients' plasma samples for circulating protein biomarkers such as Aβ and Tau (A–B). An acoustofluidic separation chip is employed for removing contaminating bioparticles and isolating AD-specific biomarkers from patient plasma (C). An acoustofluidic microreactor (D) is employed for developing functional nanoarray-based SERS (E) and electrochemical immunosensor (F).
Alzheimer's disease (AD) is a progressive and irreversible neurodegenerative brain disorder that affects tens of millions of older adults worldwide and has significant economic and societal impacts. Despite its prevalence and severity, early diagnosis of AD remains a considerable challenge. Here we report an integrated acoustofluidics-based diagnostic system (ADx), which combines triple functions of acoustics, microfluidics, and orthogonal biosensors for clinically accurate, sensitive, and rapid detection of AD biomarkers from human plasma. We design and fabricate a surface acoustic wave-based acoustofluidic separation device to isolate and purify AD biomarkers to increase the signal-to-noise ratio. Multimodal biosensors within the integrated ADx are fabricated by in-situ patterning of the ZnO nanorod array and deposition of Ag nanoparticles onto the ZnO nanorods for surface-enhanced Raman scattering (SERS) and electrochemical immunosensors. We obtain the label-free detections of SERS and electrochemical immunoassay of clinical plasma samples from AD patients and healthy controls with high sensitivity and specificity. We believe that this efficient integration provides promising solutions for the early diagnosis of AD.